Removing Highly Toxic Phenolic Compounds
Wastewater treatment options
Apr 24th 2020
Key Takeaways
- Phenol and its derivatives (phenols, or phenolic compounds) are moderately water-soluble pollutants, common to the wastewaters of various industries, including oil & gas, paint manufacturing, phenolic resin production, paper and pulp factories, and pharmaceutical industries. Phenolic compounds are used in low concentrations in disinfectants, and are also present in many alcoholic beverages, pharmaceuticals, and cosmetics.
- Excessive phenolic compounds are harmful to human health and the environment. Chlorophenols, by-products of chlorinating phenol-containing water, are carcinogens.
- Existing treatment options include biodegradation, distillation/evaporation, adsorption and extraction, membrane separation, and chemical oxidation. A treatment system needs to be chosen and engineered carefully, with consideration of specific wastewater chemistry, operating conditions, and economics.
- Recent advances in membrane separation technology can help optimize the treatment of phenolic compounds-contaminated wastewaters, making zero liquid discharge (ZLD) or minimum liquid discharge (MLD) options more cost-effective.
Uses, Toxicity, & Wastewater
Phenolic compounds are profoundly toxic to humans, animals, and aquatic life, and can also form carcinogenic chlorophenols in the presence of chlorine. They are considered priority water pollutants by the EPA and the NPRI in the USA and Canada, respectively. Many jurisdictions have strict discharge limits for phenols, typically <0.5 mg/L.
The phenolic compounds consist of an aromatic hydrocarbon group bonded to a hydroxyl group (–OH). Phenols are often moderately water-soluble and smaller phenol molecules can be volatile. Phenols occur naturally in small, relatively harmless concentrations. They are also synthesized on an industrial scale for use in disinfectants, medicinal products, and as ingredients for many polymers, resins, and rubbers for the plywood, tire, construction, automotive, and appliance industries. Small quantities are present in alcoholic beverages, pharmaceuticals, and cosmetics. Phenols can be found in the wastewaters of these industries and others, such as oil & gas.

Treatment Options for Phenols in Wastewaters
Many technologies are available for the treatment of phenols-laden wastewaters—with highly varied advantages and disadvantages. For example, some are destructive, while others allow the recovery of phenols for potential reuse. The economics of the different processes vary significantly depending on water chemistry, scale, treatment requirements, and other operating conditions. Options need to be carefully analyzed for a specific application before treatment decisions are made. Many of the available phenol treatment processes are discussed below and summarized in this table.
| Treatment | Description | Recovery | Pros | Cons |
|---|---|---|---|---|
| Bio- Degradation | Uses microorganisms to biodegrade phenols | Destructive |
|
|
| Membrane Separation | Uses high pressure & fine membranes to reject phenols, other compounds, & ions | Possible |
|
|
|
Distillation/ Evaporation |
Uses heat & difference in volatility to separate phenols from water | Possible |
|
|
| Adsorption & Extraction |
Phenols adsorb to solids such as activated carbon (AC)
Solvent extraction uses relative solubilities to separate liquids |
Adsorption: typically
unfeasible
Extraction: possible |
|
|
| Chemical Oxidation | Uses one of a variety of oxidising methods, e.g. the Fenton reaction | Destructive |
|
|
Biological and Enzymatic Degradation
Biological treatment is a highly established, successful method for the removal of phenols from water. The treatment uses either aerobic or anaerobic microorganisms to biodegrade phenols. It is relatively inexpensive, safe, easy-to-operate, and environmentally friendly. It generally produces simple, harmless products. Membrane bioreactors (MBRs) are a variant of conventional bio-degradation. They use an activated sludge process combined with a membrane filtration step to produce very high-quality water with a small footprint.
Another phenol treatment uses enzymes to catalyze phenol polymerization for precipitation from water. Depending on the circumstances (e.g. availability and cost of appropriate enzymes) it can be even more cost-effective than biological treatments.
However, both biological and enzymatic treatments are not suitable for all water chemistries, for example, high concentrations of phenols, high salinity, and high acidity. These treatments may also require interventions such as further chemical additives and aeration. This means that they are not suitable or cost-effective for all applications.

Membranes
Membrane methods use reverse osmosis (RO) membranes to reject phenols, as well as other compounds and ions. RO membranes are typically cost-effective and reliable, with low power consumption, a small footprint, and scalability. Although they have good rejection performance for many phenols, their performance can be pH dependent. Furthermore, a polishing step may help to further lower the phenol concentration to reach discharge limits, especially for smaller phenol-family molecules. They are particularly suited to treating high concentrations that are unsuitable for biological treatment.
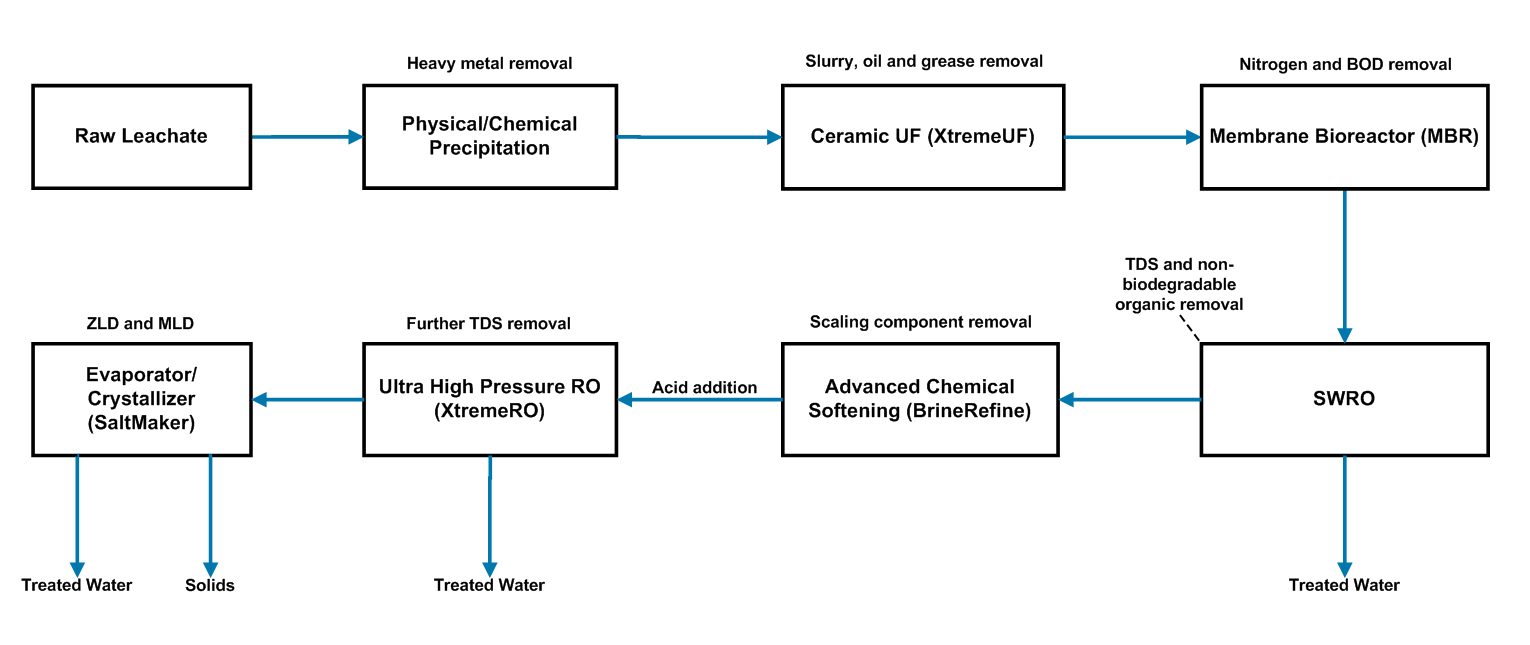
Some risks must be considered such as membrane fouling and scaling. However, comprehensive membrane treatment processes with appropriate pre-treatment can manage the risk effectively, making RO and nanofiltration (NF) very cost-effective and convenient for the treatment of phenols. Saltworks’ XtremeRO system is depicted below.

Distillation/Evaporation
A variety of evaporation/distillation options are possible for the separation of phenols from wastewater. They use the relative volatility of some phenols to purify water. Distillation methods generally have high energy costs and are typically viable for high phenol concentrations only. Due to the spread of volatility across different members of the phenol-family, such methods may not be suitable for all phenols.
Pervaporation (PV) applies a vapor pressure difference to pull phenol vapor through a membrane, exploiting the difference between the affinities of phenol and water to the membrane. With relatively low energy consumption and simple operation, it is effective for separating low volatility organics, including some phenols.

Adsorption and Extraction
Adsorption is commonly used to remove phenols, using solid consumables such as activated carbon (AC). The economics depend on the cost of the adsorbent and the cost of its disposal or recycling. Once spent, the management of phenol-saturated solids is critical and includes options for reuse or disposal. Although usable for the treatment of any concentration of phenols, adsorption is most cost-effective for removing low concentrations, making it an excellent final-step polish. The presence of other organics in the water will increase the cost since these may also occupy adsorption sites in the AC.
Solvent extraction is a similar, liquid-liquid process. It is standardized and non-destructive for phenols compounds, useable over a wide range of concentrations. However, it is only cost-effective in some circumstances, typically on a small scale.

Oxidation and Fenton’s Reaction
Oxidation is a destructive method for the treatment of phenols and other organics. It can use a variety of conventional oxidizing agents (ozone, chlorine, permanganate, etc.), catalysts, and conditions, including irradiation. The choice of oxidation strategy depends heavily on the economics, wastewater chemistry, and other conditions. Costs are typically low to moderate, and oxidation can be used in varied operating conditions. Reaction products may require further treatment or be precipitated (meaning that consideration may need to be given to appropriate solids management).
The Fenton reaction (and variants) use H2O2 and Fe2+ to produce hydroxyl radicals, which oxidize organics such as phenols. As a phenol treatment method, it is usually cost-effective, and suitable for high concentrations. However, it is pH dependent and may not suit all water chemistries. As with many other chemical treatments, sludge is produced that requires management.
Contact Saltworks for Phenols Solutions
Saltworks’ process experts support our clients by providing options analysis—we help you to decide on the right treatment for your phenols-laden wastewater. Providing you with the right solution is what matters most, so our recommendations may include solutions from third-party vendors rather than our own products (or combinations of both). We have extensive experience in developing specifications for third-party vendors for our clients. Given our position in the water industry, we know who builds the best sub-processes and process elements.
Saltworks offers two ‘in-house’ solutions for phenols that are adaptable for new or existing treatment chains:
- Option 1 uses reverse osmosis/nanofiltration, combined with our ultra-high pressure XtremeRO system to remove most of the phenolic compounds. We optionally add polishing to remove the rest cost-effectively. Option 1 is better suited for low-TDS wastewater.
- Option 2 uses our SaltMaker evaporator crystallizers to reduce the volume of high-TDS wastewater to minimal or zero liquid discharge. Post-treatment systems can polish the phenol-containing condensate. Option 2 is better suited for high-TDS wastewater.
Once a treatment technology is chosen, we can bring our testing, automation, and piloting expertise to the project. Get in touch to see how Saltworks can help you to meet your phenols challenges.

About Saltworks
Saltworks Technologies is a leader in the development and delivery of solutions for industrial wastewater treatment and lithium refining. By working with customers to understand their unique challenges and focusing on continuous innovation, Saltworks’ solutions provide best-in-class performance and reliability. From its headquarters in Richmond, BC, Canada, Saltworks’ team designs, builds, and operates full-scale plants, and offers comprehensive onsite and offsite testing services with its fleet of mobile pilots.
Related Resources

Zero Liquid Discharge (ZLD) and Minimal Liquid Discharge (MLD) Become Lower Cost Through Reverse Osmosis Innovation
Saltworks is pleased to announce that recent advancements in reverse osmosis technology are now commercially available. Reduce conventional RO brine volumes by 50%, achieving brine concentrations of 130,000 to 150,000 mg/L TDS with spiral wound RO membranes.

Ultra High Pressure Reverse Osmosis for Landfill Leachate
Reverse osmosis (RO) is the best available technology to treat landfill leachate for surface discharge. Possible trace volatile organic compounds (VOCs) and ammonia emerging in the RO permeate can be removed with a polishing step to meet the highest discharge standards.

Pharmaceutical Industry Wastewater Treatment
No two pharmaceutical wastewaters are the same. Saltworks can help you to treat complex wastewaters from this critical industry with certainty. We implement solutions that target organics, metals, and ions for removal, reduce brine waste, and allow water reuse.

Anaerobic Digester Wastewater Management During Biogas Production
Digester wastewaters are by-products of biogas production in anaerobic digesters. They require treatment prior to disposal. To meet regulation compliance, treatment options range from minor interventions such as selective contaminant removal, to major interventions such as minimum and zero liquid discharge (MLD/ZLD).