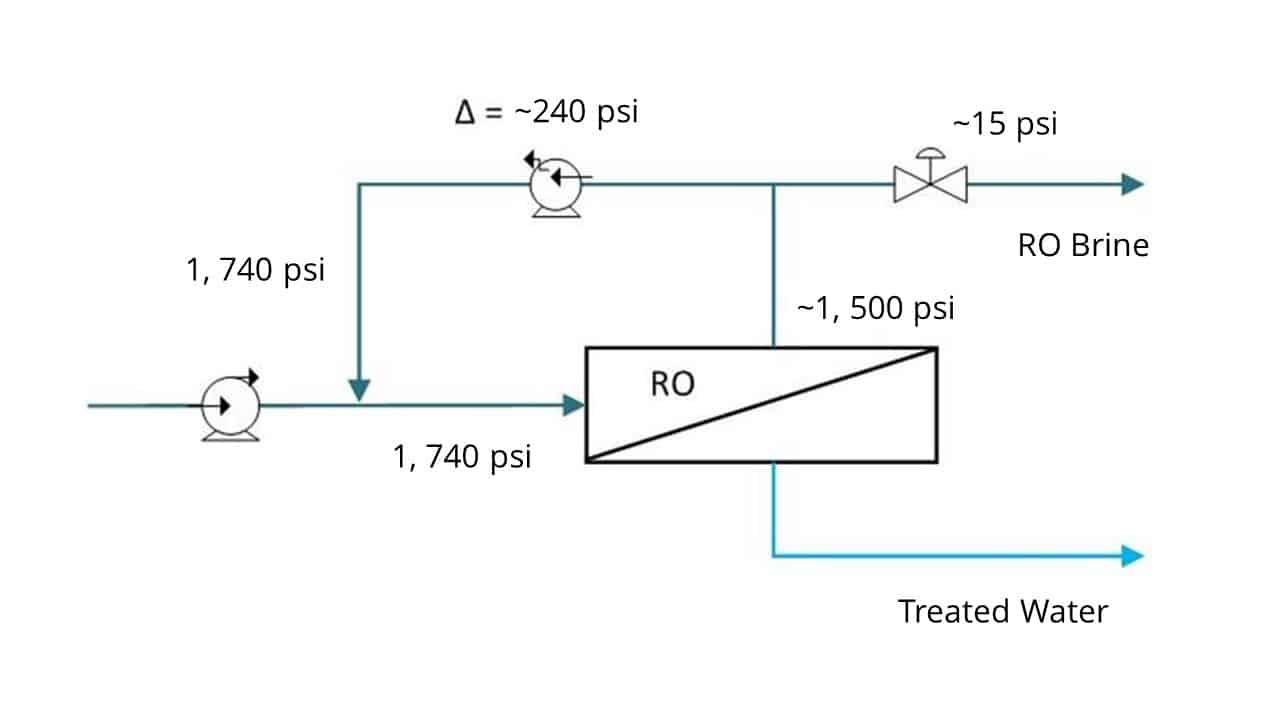
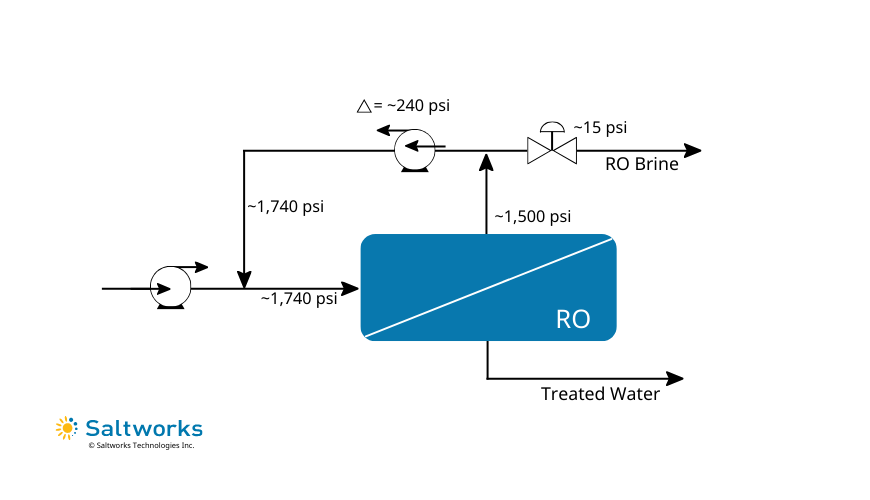
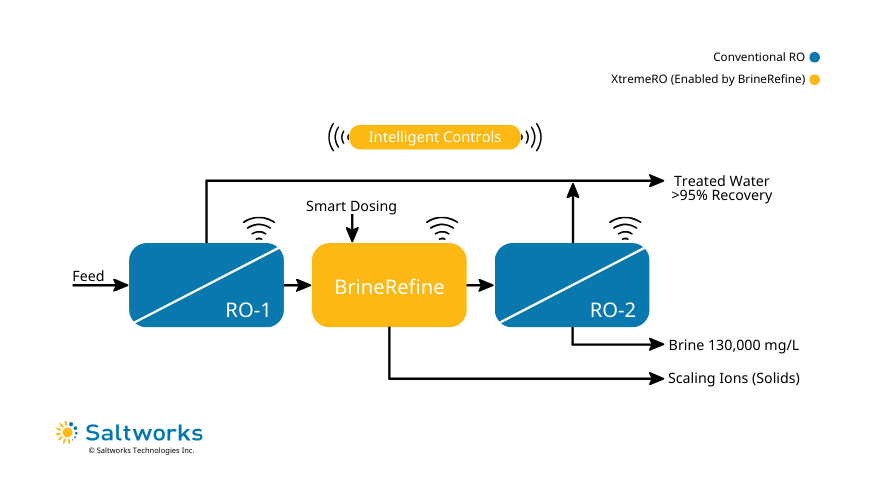
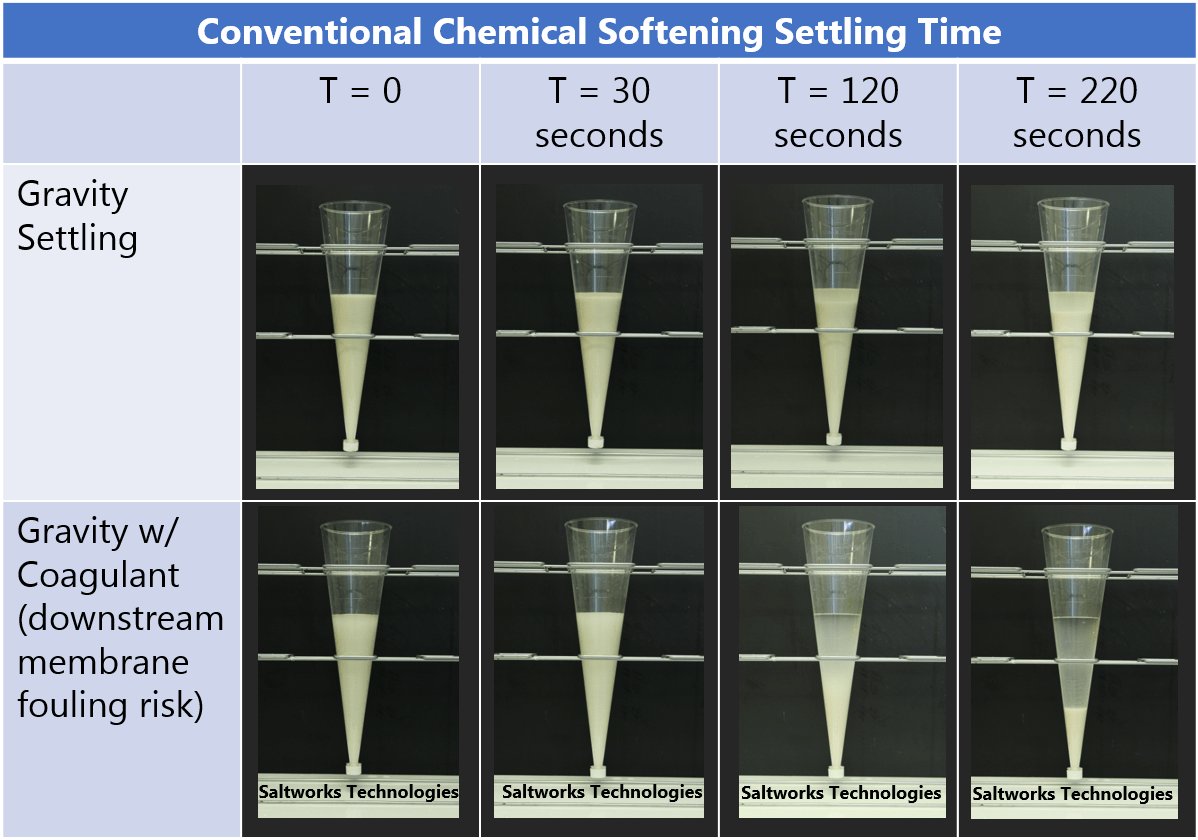
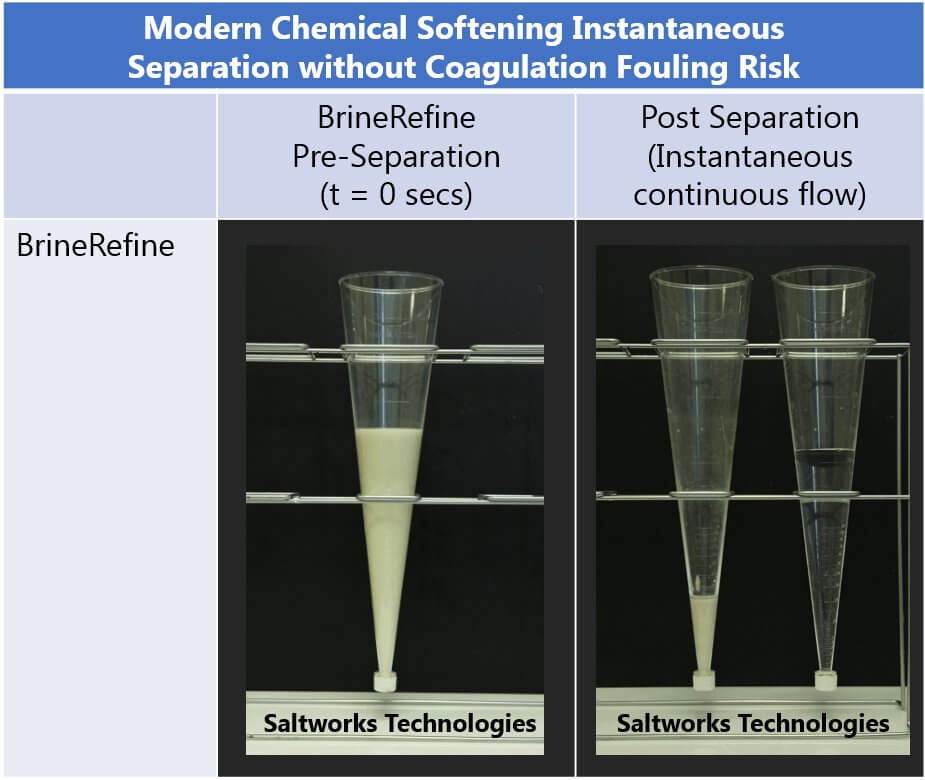
FlexEDR Selective FGD Wastewater Pilot Test Results
Mar 19th 2019
Project Summary
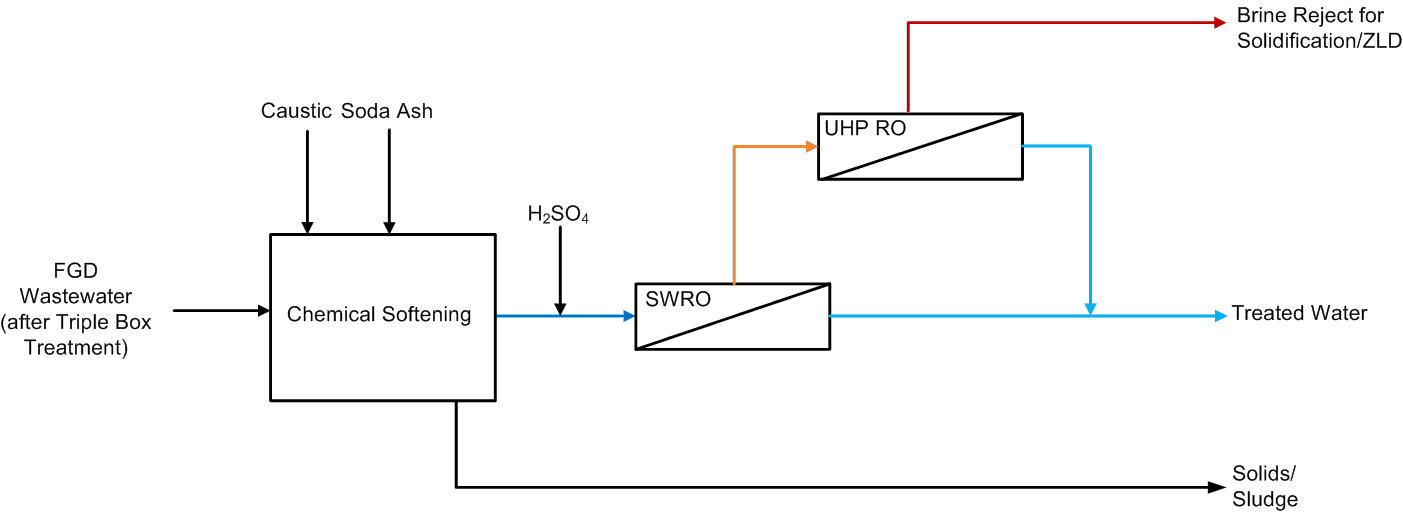
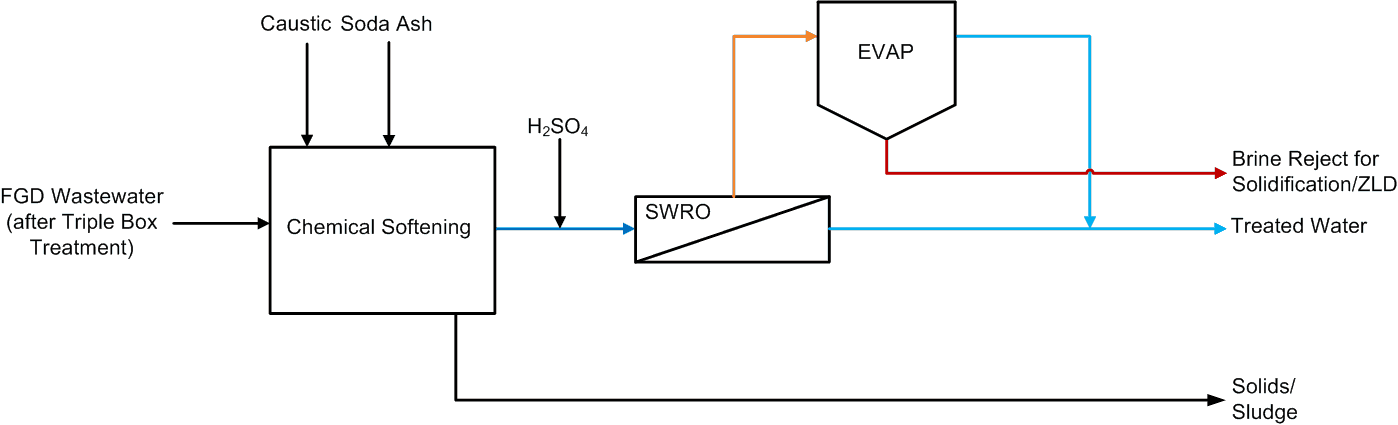
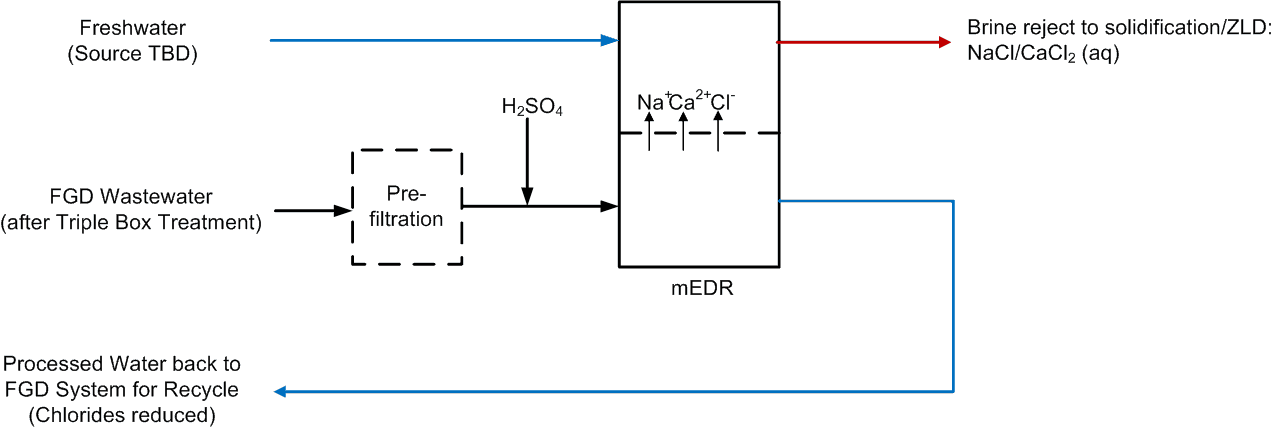
Saltworks Technologies Inc. (Saltworks) completed an off-site FlexEDR Selective pilot test to treat flue gas desulfurization (FGD) wastewater from a coal fired power plant in China. The objective was to reduce chlorides such that the FGD wastewater could be internally recycled and final treatment costs reduced notably. FGD wastewater is generated and released primarily due to chloride content that inhibits sulfur absorption in the stack and can cause corrosion problems. If chlorides can be removed, the FGD wastewater can be recycled.
The results showed that Saltworks FlexEDR Selective achieved:
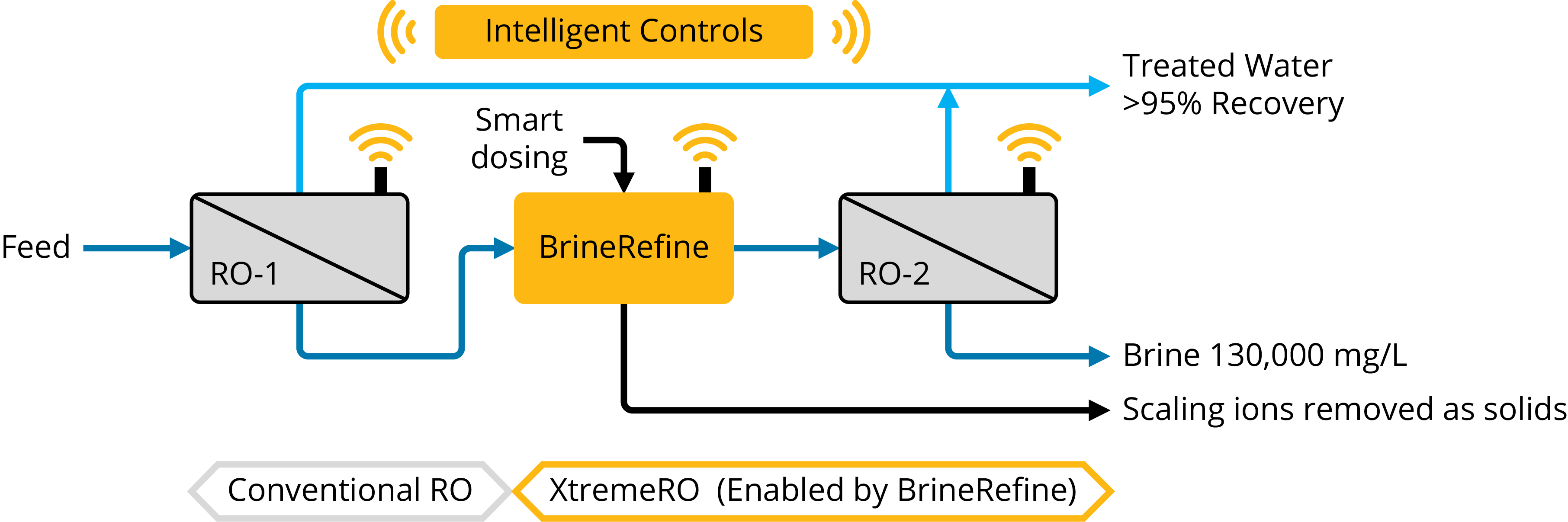
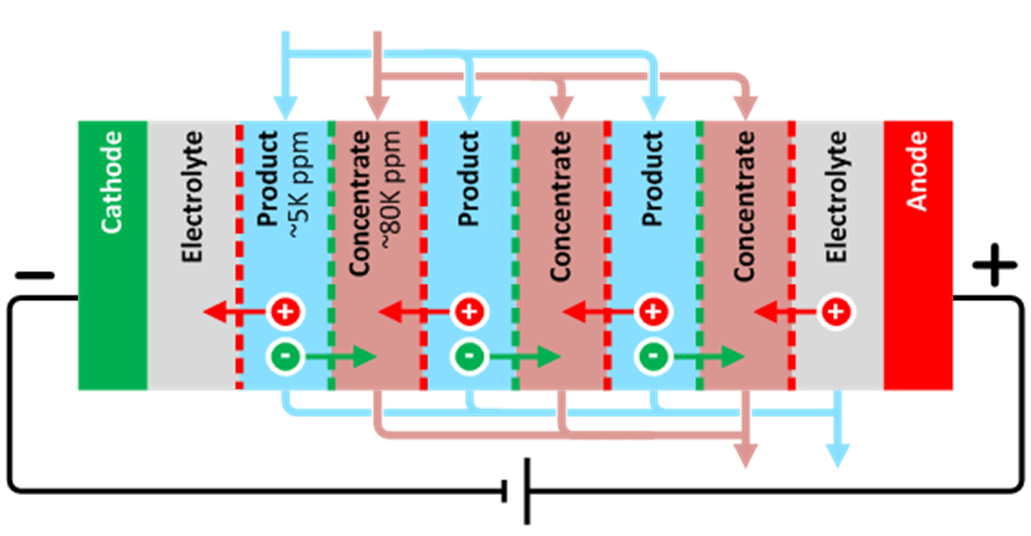
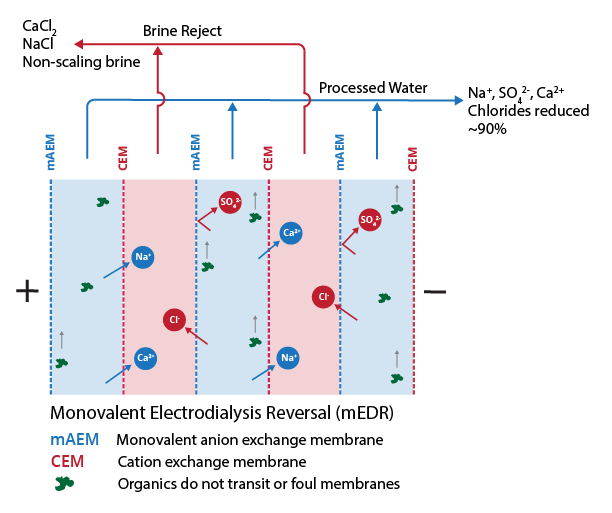
- Reliable treatment built from the second most common membrane desalination technology – electrodialysis reversal (EDR) – with modernized membranes and process controls.
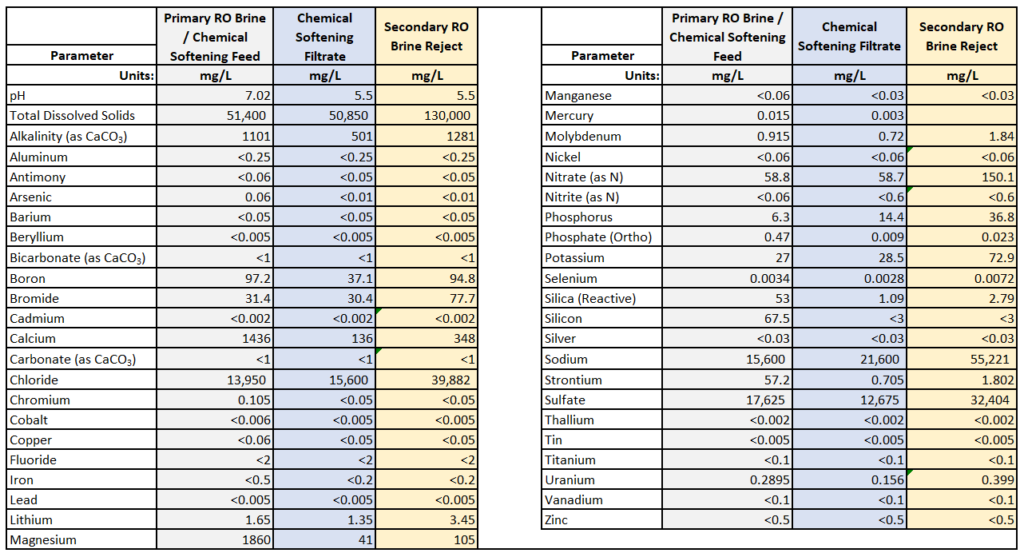
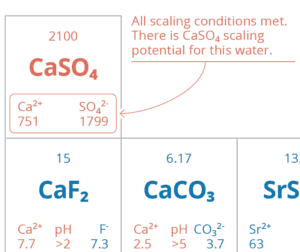
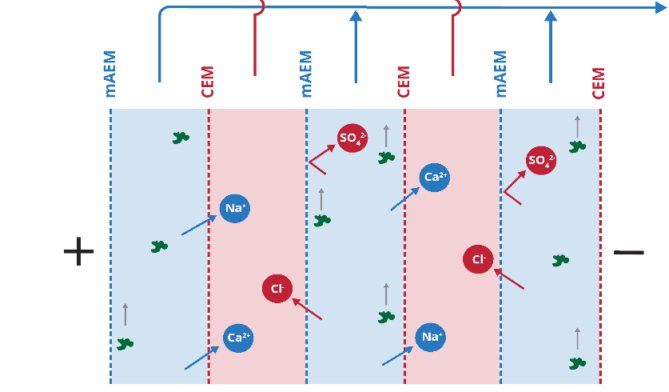
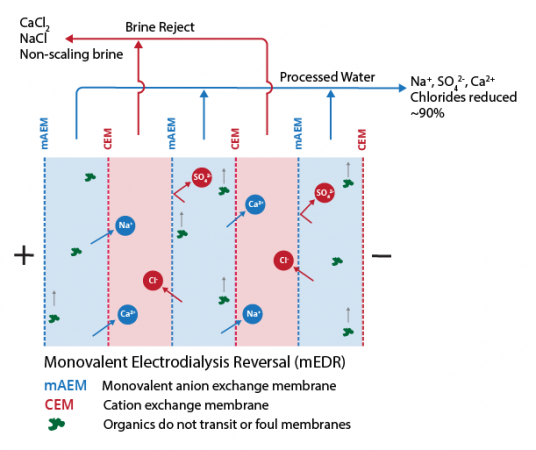
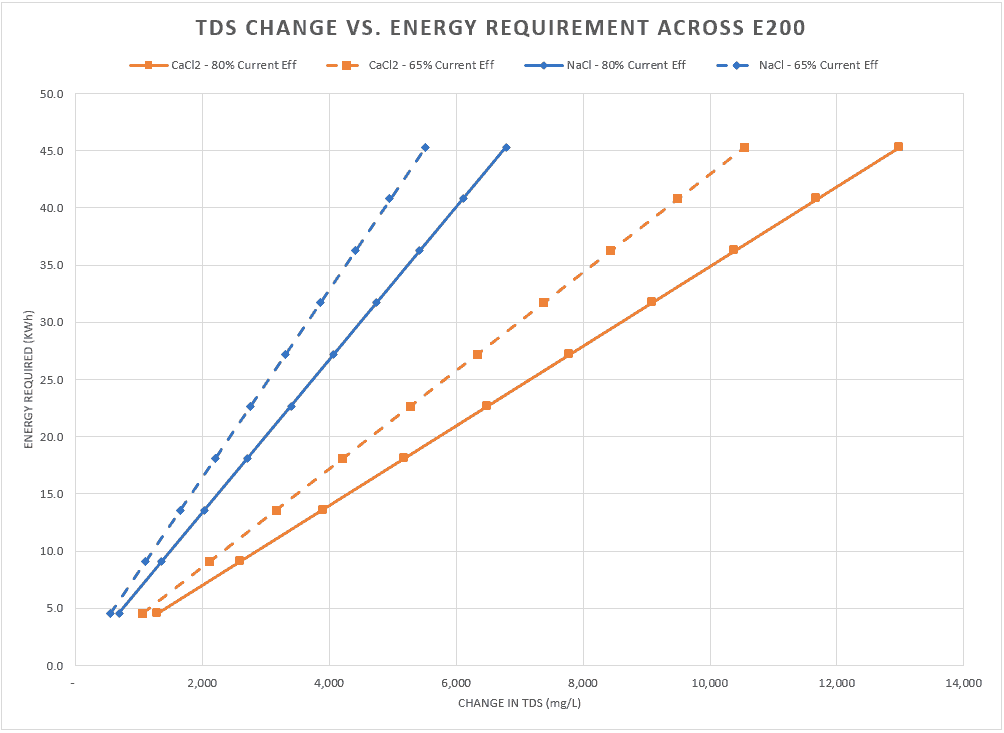
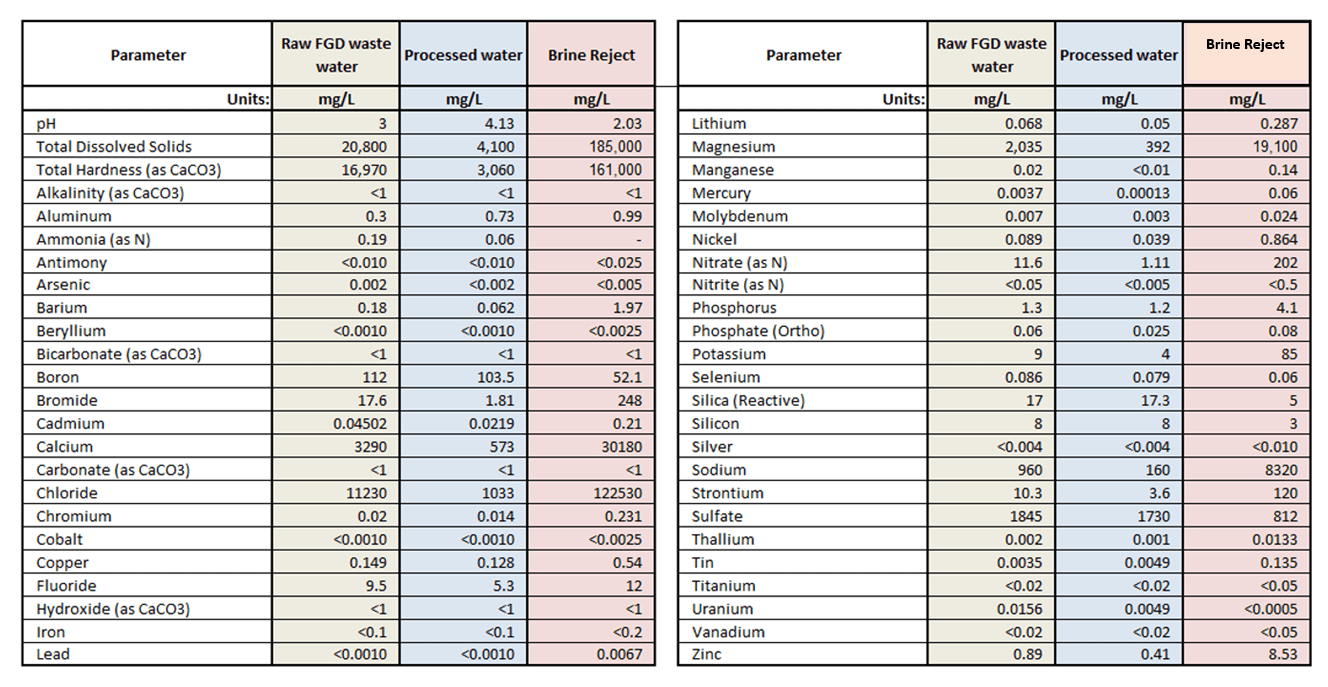
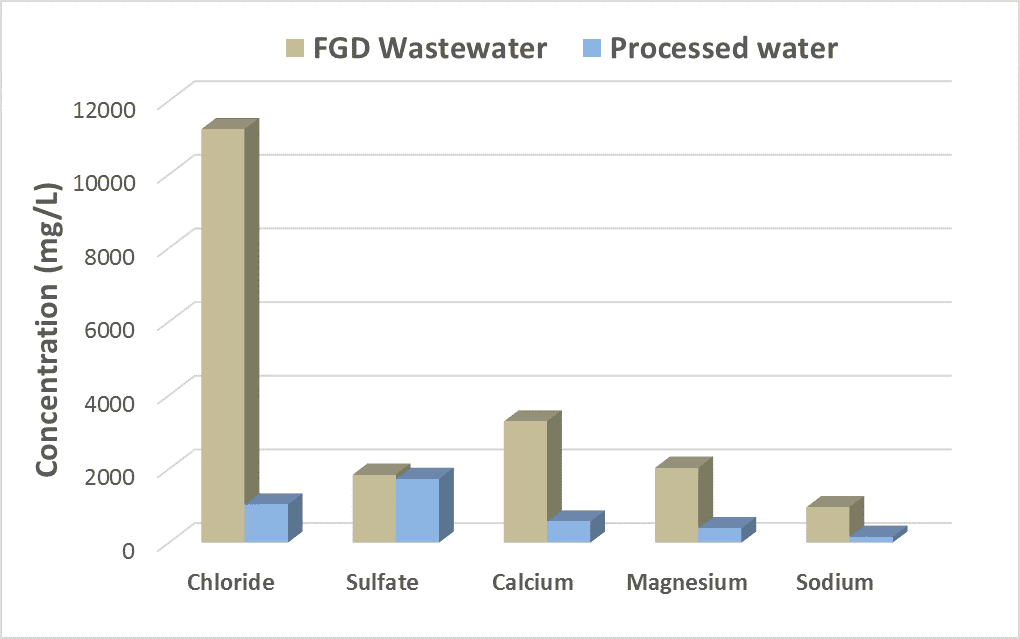
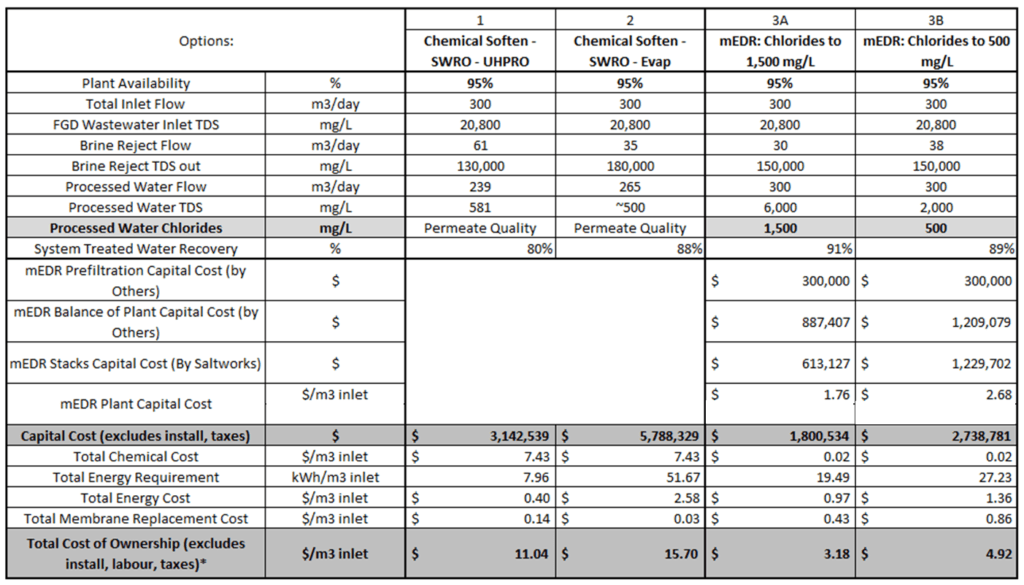
- 90% recovery on a highly scaling calcium sulfate FGD wastewater without the need for expensive soda ash softening. The system produced a predominantly calcium chloride brine with 127,000 mg/L total dissolved solids (TDS) concentration. The brine could be directly solidified with fly ash and other solidification agents for zero liquid discharge, eliminating the need for a crystallizer.
- Removed 78% of the chlorides with ease: treated water from ~6,900 mg/L chlorides to less than 1,500 mg/L, such that it could be recycled back to the FGD system.
For the complete results of the pilot including detailed water chemistry and cost, download the free case study below.
Download the free FGD wastewater pilot results
About Saltworks
Saltworks Technologies is a leader in the development and delivery of solutions for industrial wastewater treatment and lithium refining. By working with customers to understand their unique challenges and focusing on continuous innovation, Saltworks’ solutions provide best-in-class performance and reliability. From its headquarters in Richmond, BC, Canada, Saltworks’ team designs, builds, and operates full-scale plants, and offers comprehensive onsite and offsite testing services with its fleet of mobile pilots.
Related Resources

How to Manage Brine Disposal & Treatment
The many options for managing brine, a term for saline wastewater from industrial processes, fall under two categories: brine treatment and brine disposal. Brine treatment involves desalinating the brine for reuse and producing a concentrated brine (lower liquid waste volume), or residual solids (zero liquid discharge).

How to Improve FGD Economics with Chloride Removal using Selective EDR
As regulations on FGD wastewater tighten, additional treatment is required. Often, this is chemically intense, and high cost. The best means to lower treatment costs is to reduce the volume of wastewater generated, usually by increasing internal recycle.

First-of-Its-Kind Coal FGD Wastewater Treatment Pilot Deployed
Saltworks has shipped a novel FlexEDR system to the southern United States that will clean up coal fired power wastewater. Flue gas desulfurization (FGD) wastewater is a byproduct of sulfur scrubbers, installed to prevent acid rain from coal plants.
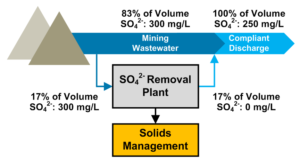
Sulfate Discharge: Measurement and Cost-Optimized Removal
There are many sulfate treatments available, with different advantages and disadvantages. Factors for consideration include capital and operational costs, solid vs. liquid brine reject for disposal, if the need is seasonal or year-round, and suitability for adverse operating conditions.