RO Brine Treatment: Why Maximizing Reverse Osmosis Recovery Matters
Jun 27th 2018
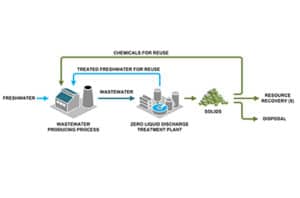
Treating RO brine should be approached from two directions. First, maximize the recovery of lower cost membrane systems to minimize the volume for later steps. Second, weigh brine disposal options against thermal treatment technology to determine whether you need to concentrate your brine or treat it down to solids.
Reverse osmosis (RO) is the most widely used saline wastewater treatment technology for a good reason: it is a cost-effective option for removing contaminants from water. This article will touch on how to minimize and manage RO concentrated brine.

Key Takeaways
- RO is highly effective and low cost, if correctly implemented.
- Start with a detailed water chemistry analysis and try to capture variability in high and low flow.
- Design a flexible treatment process that maximizes RO system recovery while avoiding scaling and fouling, and maximizing performance and reliability.
- You can often treat saturated RO brine further with intelligent membrane systems, by removing scaling ions and using RO for a second time.
- Evaluate your brine disposal options and complete a cost trade-off assessment for each one.
- Look ahead to a future where your brine disposal options could change, and plan for those possible changes.
- Contact Saltworks if you need help with any of the above.
Start with the Fundamentals: What is Your Water Chemistry?

Getting the most out of an RO system is easier when you are equipped with a comprehensive analysis of your water chemistry. This will provide essential information for the design stages, such as your scaling ions, and prepare you for any treatment challenges. Take samples during periods of high, low and normal flow and send them to a water analysis lab or to our analysis team to get help with understanding the range in your water chemistry.
Stop Fouling & Scaling on RO Membranes Before It Starts

With your water chemistry analysis in hand, devise a treatment strategy that prevents performance reductions from scaling or fouling. If scaling ions are ignored, your RO could rapidly scale or operate at low recovery, leaving performance on the table. If you plan for scaling ions, RO can be extremely reliable and optimized for performance. There are solutions for removing scaling ions. These range from the simple, such as pH control and anti-scalants, to more advanced solutions, such as selective removal of scaling ions and live monitoring of RO-normalized flux.
Choose an RO System that Can Achieve Your Performance Targets
Not all RO systems are created equally. Many factors can affect the potential recovery in your treatment process, which include:
- Process controls and intelligence to monitor and correct performance: If water chemistry varies, the RO performance should also be adjusted. Do not ‘set and forget’ your RO system.
- Concentrating, purging, and flushing cycles to wash away any scaling or fouling before it becomes irreversible.
- Clean-in-place systems, matched to your water chemistry.
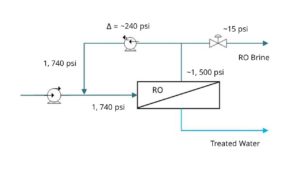
- Recovery demands, which will determine pressure.
- Potential accumulation of organics.

When you consider building an RO system, consult with water treatment experts who can help you determine the setup that is best suited to your needs. While RO is widely available in low cost systems, and these systems may fit your application, there are really only two categories of RO: basic and advanced. Basic RO works well for simple waters but breaks down rapidly when pushing brine concentrations. Advanced RO can push limits without compromising performance. Only pay for advanced performance if you need it, such as when you require brine volume reduction and high recovery.
It is important to factor in the costs of downstream treatment or disposal technologies as well as upstream pre-treatment when making your business decision to invest in an RO plant.
Maximize RO System Recovery Before Looking to Thermal Systems
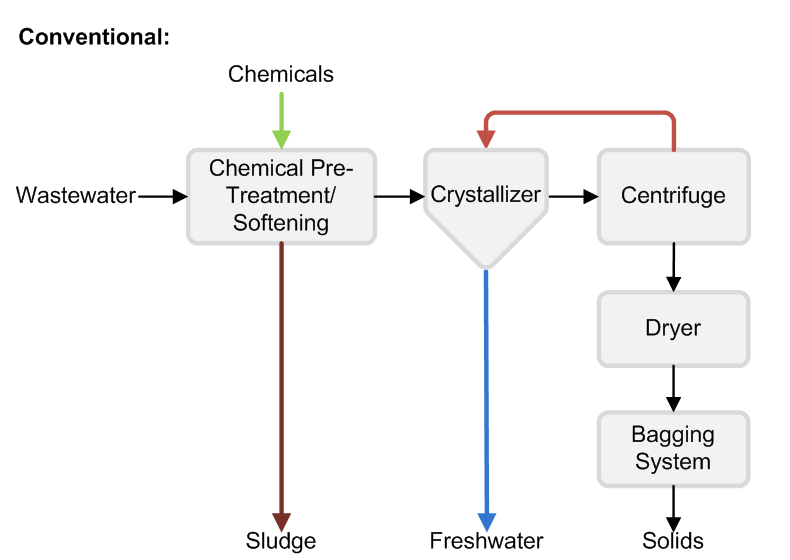
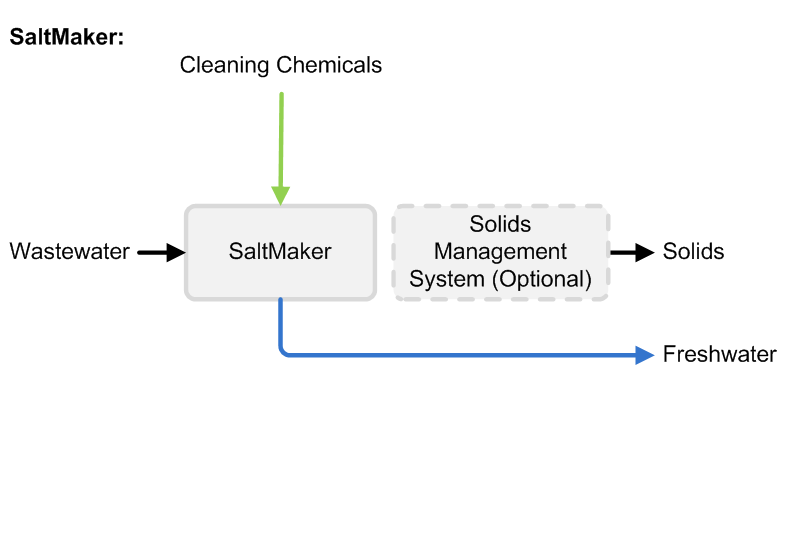
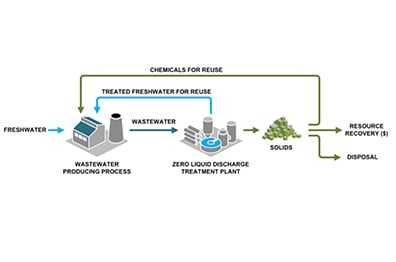
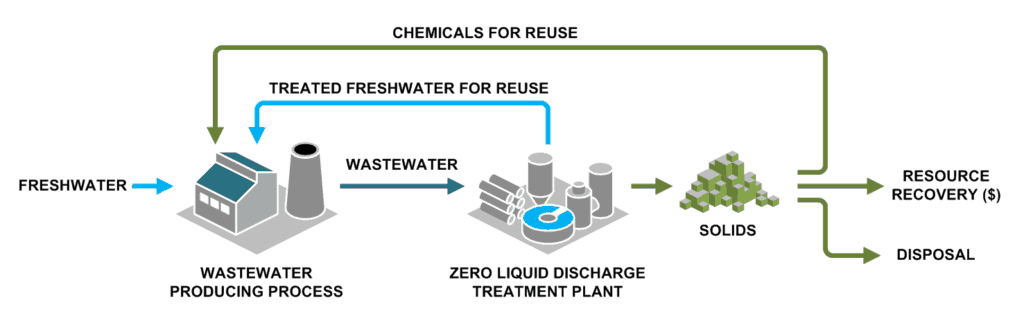
The priority for some RO system operators is to minimize the brine volume they must treat or dispose. Treating concentrated RO brine to reduce the disposal volume further or produce zero liquid discharge solids can substantially increase capital and operating costs and requires equipment with a larger physical footprint, such as evaporators and crystallizers.
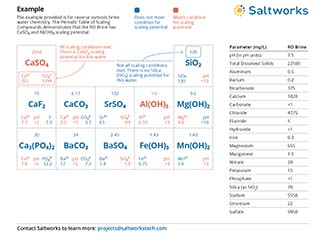
Often, concentration of RO brine is limited by scaling compounds, such as CaSO4 or SiO2 and other low-solubility compounds. If these scaling compounds are removed, or separated, the recovery of membrane systems can increase and reduce the volume of brine.
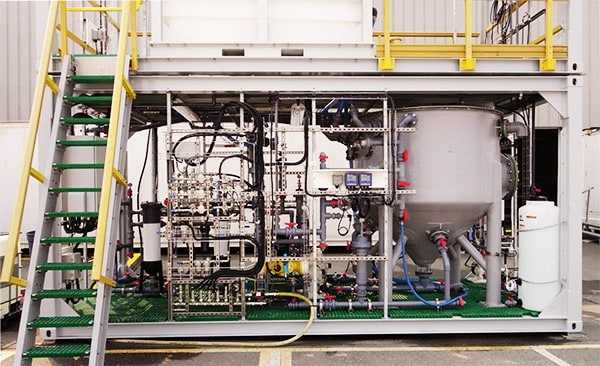
To reach the highest concentration levels with your RO brine, trust intelligent chemical softening systems like Saltworks’ BrineRefine. Most RO systems will hit their treatment limits near 50,000 mg/L total dissolved solids (TDS). BrineRefine removes scaling ions from the water, and enables the use of ultra-high pressure RO systems, which can reach up to 130,000 mg/L TDS brine concentrations.
Another option to maximize brine concentration is through selective ion removal with electrodialysis reversal (EDR), which can concentrate brines up to 180,000 mg/L TDS.
Trust Intelligent Automation Over Manual Management
The most advanced RO systems offer automation and systems controls that can adapt to changing water chemistries. One example is automated flux control—an intelligent RO that will increase or decrease permeate flux as it detects changes in scaling compounds. This will minimize fouling in the presence of high concentrations of scaling ions and maximize recovery when concentrations are low.
If you choose to use an RO system that requires more monitoring and manual performance adjustments, be prepared to invest more in maintenance and operating costs over the long run.
Evaluate Your Brine Disposal Options
Regardless of the RO system you choose, the concentrated RO brine needs to be managed via disposal or further treatment. There are a range of brine disposal options and their availability will depend on a variety of factors. If your brine disposal pathways are limited or cost-prohibitive, your next option is to evaluate further concentrating your brine using thermal treatment systems.
Plan Your Process with Downstream Thermal Systems in Mind

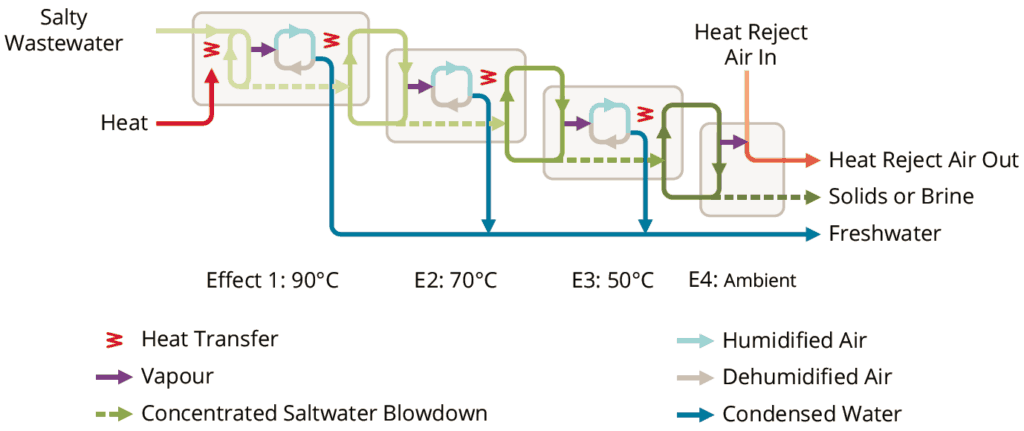
Concentrating RO brine even further requires thermal systems, such as evaporators or crystallizers. Although they cost more than RO systems, thermal systems can reduce RO brine to zero liquid discharge solids. Maximizing upstream RO recovery will minimize the size and operating cost of your thermal treatment steps.
This can be done by using a treatment strategy that minimizes the use of chemical pre-treatment; any chemicals added upstream in a treatment process will cause greater impacts on overall economics further downstream. You can avoid adding chemicals by choosing treatment technologies that do not rely on extensive pre-treatment, such as intelligent RO systems or EDR with membranes that resist organics. Alternatively, you can use BrineRefine’s intelligent chemical softening for precise dosing to both prevent scaling and avoid chemical overdosing.
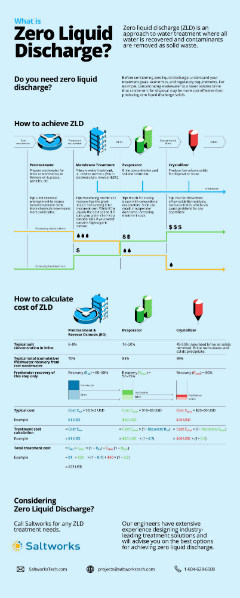
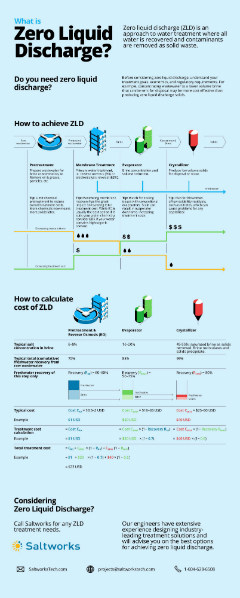
Treating RO Brine: To Concentrate or To Crystallize?
The options for treating RO brine depend on whether you need zero liquid discharge or a reduction in brine volume. Download our infographic to further explore the roles of different treatment technologies for brine.
If you do not have a disposal outlet available or disposal costs more than $20/m3, concentrating brine with an industrial evaporator can offer advantages. Crystallization is usually the most expensive step in a water treatment train and should only be considered if you need to produce solids, or face disposal costs higher than $40/m3.
Look to the Future: A Rising Tide of Brine Treatment Regulations
As increasing focus is placed on water, have you considered making your treatment facility future-proof before investing? Evaluating and building in options for expansion and change are low cost while still at the drawing board. Your brine disposal costs could increase if you use a third party, you may require additional capacity, your water could change as a result of upstream production, or you may need to meet new regulatory targets.
To reduce future risks that might arise from such changes, evaluate your options and future scenarios before making an investment. Reach out to the water treatment experts at Saltworks.
About Saltworks
Saltworks Technologies is a leader in the development and delivery of solutions for industrial wastewater treatment and lithium refining. By working with customers to understand their unique challenges and focusing on continuous innovation, Saltworks’ solutions provide best-in-class performance and reliability. From its headquarters in Richmond, BC, Canada, Saltworks’ team designs, builds, and operates full-scale plants, and offers comprehensive onsite and offsite testing services with its fleet of mobile pilots.
Related Resources

First Commercial Order of Next Gen UHP RO
Saltworks is thrilled to announce the first full-scale order for an industrial Ultra-High Pressure Reverse Osmosis (UHP RO) system with Nitto high performing HYDRANAUTICSTM PRO-XP1 spiral wound membranes rated to 1,800 psi (124 bar).

RO & Evaporator Scale Control: The Guide to Better Performance
Scale is a crust that forms on membranes, heat transfer surfaces, and on the inside of pipes as salts precipitate out of solution. It blocks flow, disrupts heat transfer, and increases energy requirements for water treatment systems

How to Manage Brine Disposal & Treatment
The many options for managing brine, a term for saline wastewater from industrial processes, fall under two categories: brine treatment and brine disposal. Brine treatment involves desalinating the brine for reuse and producing a concentrated brine (lower liquid waste volume), or residual solids (zero liquid discharge).

XtremeRO Reverse Osmosis & Nanofiltration
Our XtremeRO and OARO provide industry-leading recovery and reliablity. Concentrate brine, reduce discharge volumes, recover freshwater and more.