As higher recoveries are pushed on RO systems, scale becomes the primary bottleneck to recovery improvements. As discussed in this RO blog, there have been many innovations within the RO field that seek to manage scale through operational ‘tweaks’ in order to achieve higher recoveries. Saltworks builds such RO systems; we understand that operational tweaks and anti-scalants do not remove the underlying risk, but rather delay the symptoms. Operating at or beyond saturation limits means that any misstep could result in membrane fouling, decreased capacity, higher energy costs, plant downtime, and more repairs. RO systems may also not be operated at their peak performance, reducing membrane recovery and wasting money on expensive brine treatment downstream of the process.
Before spending money to remove ions that cause scale, consider which of the three categories you may fit into:
1. Basic RO: Desired recovery can be achieved with basic RO and a sound anti-scalant program (lowest cost).
2. Advanced RO: Applies operational changes, such as cycling concentrations while draining some brine, high cross-flow, automated flushes and clean-in-place cycles (CIPs), each of which may push an RO to its recovery goal. Alternatively, a scaling risk may limit the brine concentration and prevent the maximum achievable recovery, which can add system costs and complexity.
Knowledge tip: Maximum RO recovery is defined by osmotic pressure limits (the pressure at which water stops permeating through the membranes). New generation RO membranes can operate up to 1,800 psi, or concentrate non-scaling fluids to 130,000 mg/L (NaCl) and 150,000 mg/L (Na2SO4).
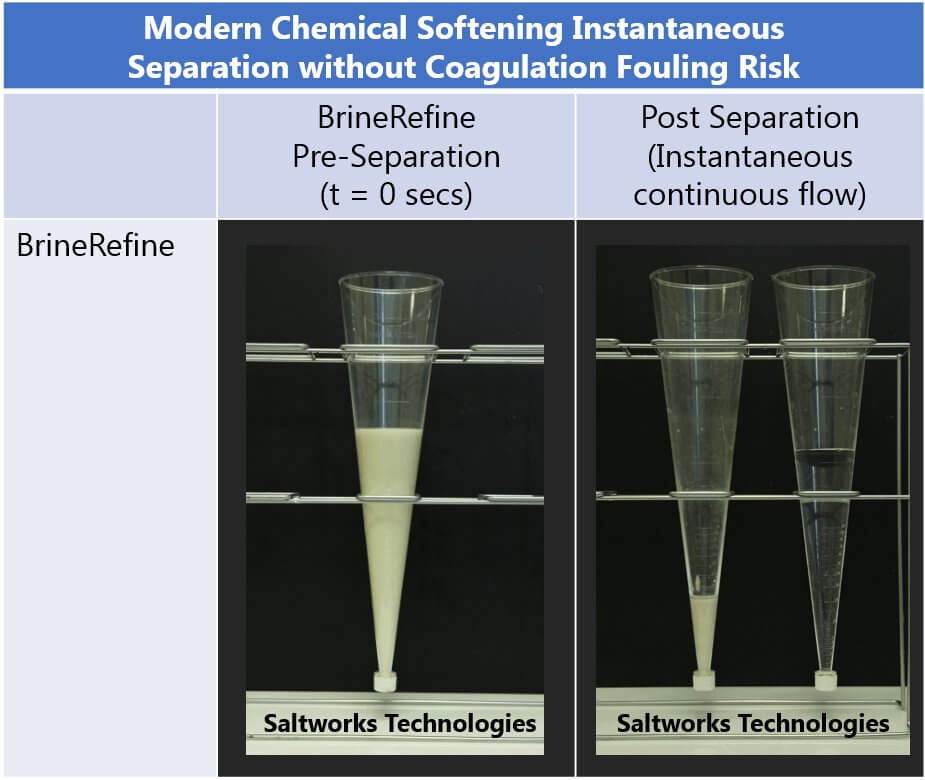
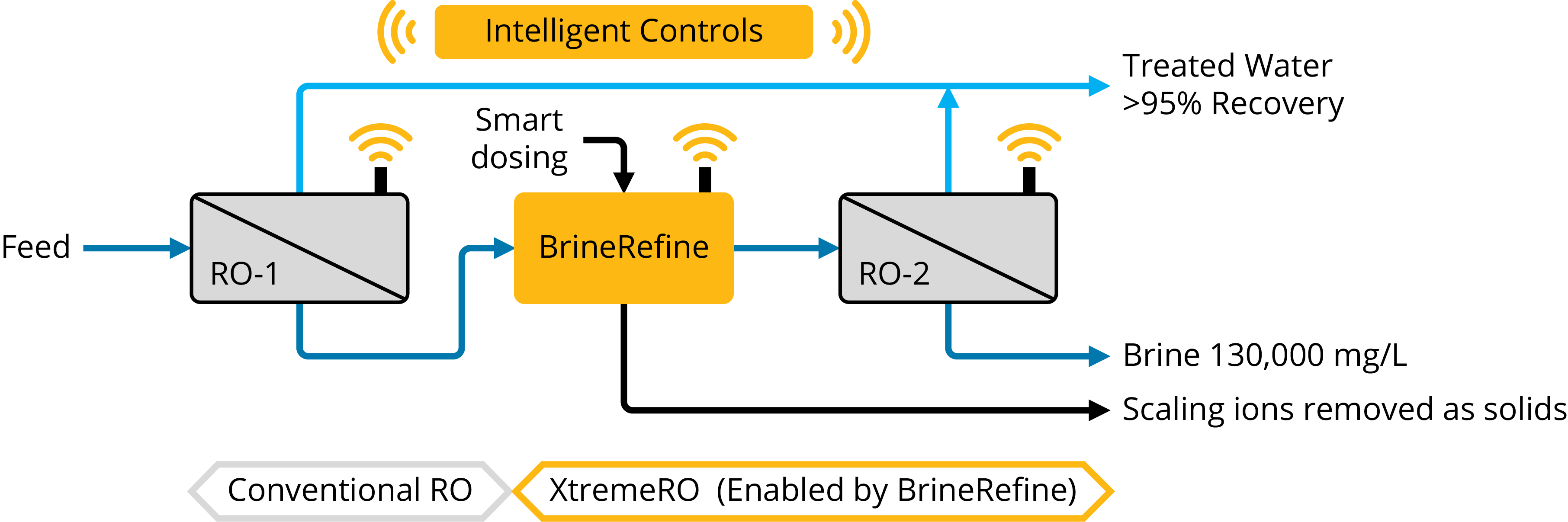
3. Chemical Softening + RO: If done correctly, chemical softening can now maximize an RO system’s recovery to reach osmotic pressure limits. Scale causing compounds are removed by chemical softening, as reviewed below, which also reduces the operational risk of membrane damage. As a note of caution, only the correct amount of scaling ions should be removed; excessive dosing will result in higher chemical costs and harmful sludge generation. Chemical softening typically adds between $2 and $5/m3 to capital and operating costs, so it is only a worthwhile investment if the added costs of chemical softening and a secondary RO are lower than the alternative brine disposal or thermal brine concentration costs (typically a minimum of $20/m3).
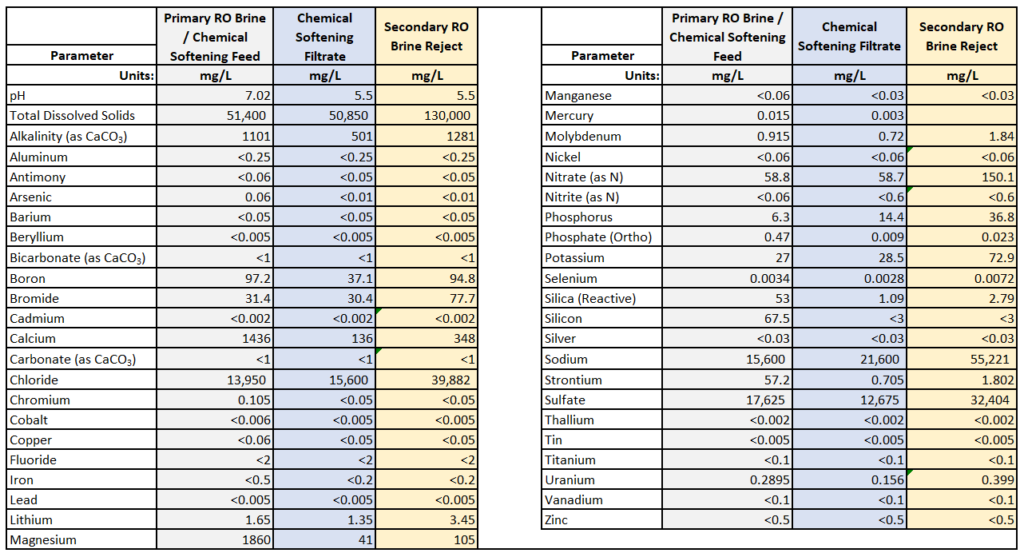
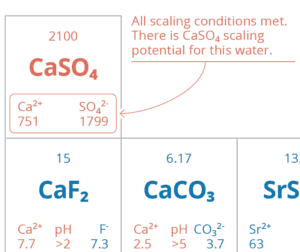
Design tip: Assess the RO brine and only remove the right amount of scale. We aim for 85% scaling potential of the downstream RO brine (with 100% representing the onset of precipitation).
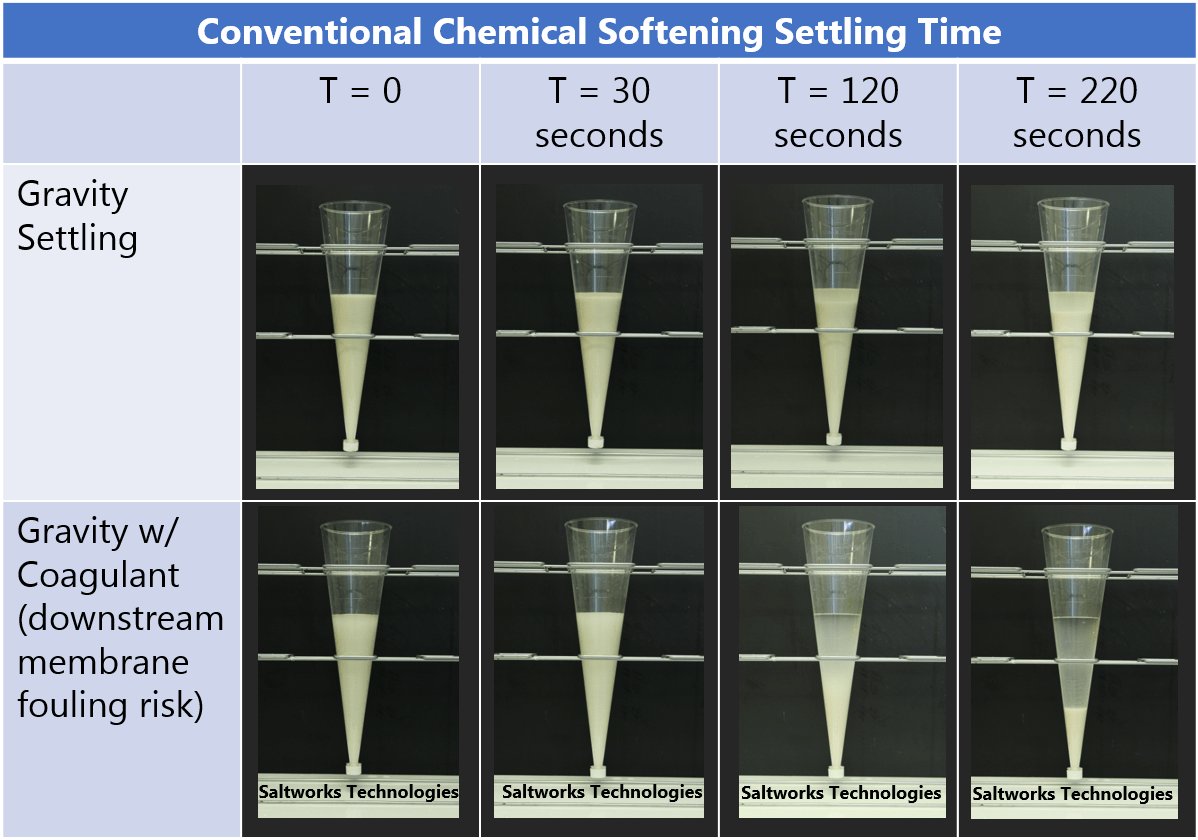
Correctly applying chemical softening can remove scale and will eliminate/mitigate the fundamental risk of precipitation on a membrane surface, allowing downstream systems to operate at high recoveries and greater reliability. Scale removal is most commonly carried out via chemical softening.
It is important to acknowledge that as water is concentrated, organics that can foul an RO will also concentrate. In more severe cases, solvents concentrate to where they become insoluble and damage the RO membrane. Consideration of an organic removal ‘kidney loop’ to treat out organics as they concentrate may be warranted. Nevertheless, do not fret too greatly about the organics, as assessments can be completed. Contact Saltworks for more details.