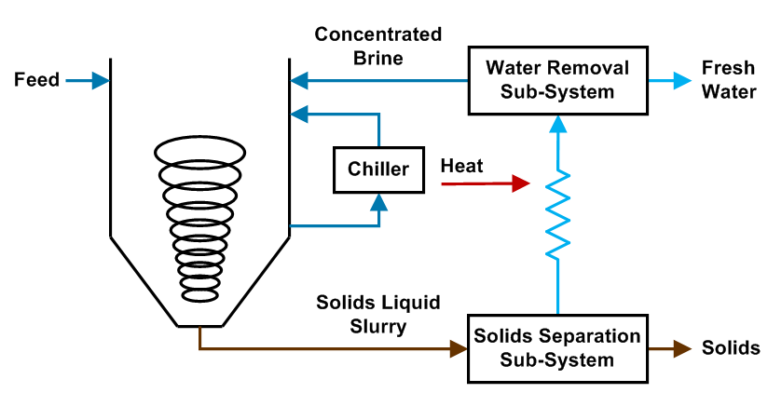
Eutectic chilled crystallization precipitates specific salt-ion pairs at low temperatures. SaltMaker ChilledCrys is an energy efficient alternative to thermal evaporators-crystallizers for select water chemistries.
Eutectic Chilled Crystallization
SaltMaker ChilledCrys uses eutectic chilled crystallization (ECC). ECC cools a fluid, forcing salts to precipitate.
If a salt-ion pair in a concentrated brine has a steep temperature-solubility relationship (e.g. Na2SO4), then cooling will form salt precipitants. The salt can be separated out while the brine is recycled for further processing.
Some salts produce hydrated crystals (e.g. Na2SO4⋅10H2O) which further remove water during the crystallization process.

ECC vs. Freeze Crystallization
ECC differs from freeze crystallization (FC) which was worked on extensively in the 1950s and 60s.
In FC, small ice particles, known as frazil, are formed. Salt is rejected from the ice during freezing. The ice is then separated, washed, and thawed leaving pure water behind.
When to Use SaltMaker ChilledCrys?
Chilled crystallization may be a viable option for zero liquid discharge (ZLD) or minimal liquid discharge (MLD) if your water consists mainly (>90%) of the following ion pairs: sodium/potassium sulfate or sodium/ammonium carbonate/bicarbonate.
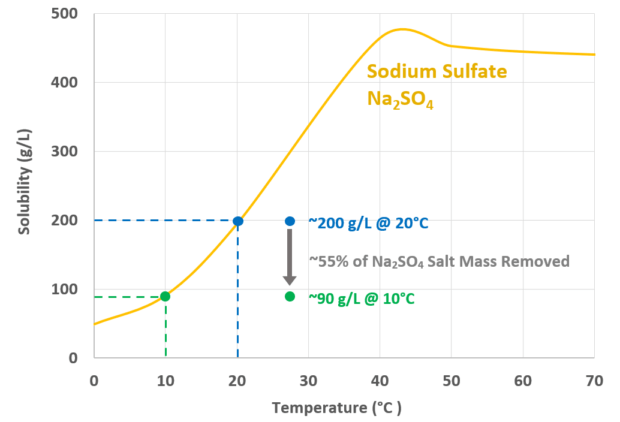
For example, cooling a solution of 200,000 mg/L sodium sulfate from 20°C to 10°C will precipitate over half of the sodium sulfate as a solid, which is easily separated.
The result is a solution with a final concentration of 100,000 mg/L sodium sulfate and the ~50% of the salt as a solid. Every solution is different, and our engineers can complete an assessment for yours.
Energy Efficient
Although SaltMaker ChilledCrys ECC only works for specific salt-ion pairs (e.g. Na2SO4), it leverages the efficiency of Carnot cycles (1 unit of power equates to 3 units of heat), making it an energy-efficient option for some projects.
Saltworks’ proprietary technology completes the chilled crystallization process in a closed loop. We package this with intelligent controls resulting in an energy- and cost-efficient MLD or ZLD solution.
Contact us today to get started on your project.