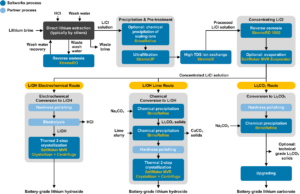
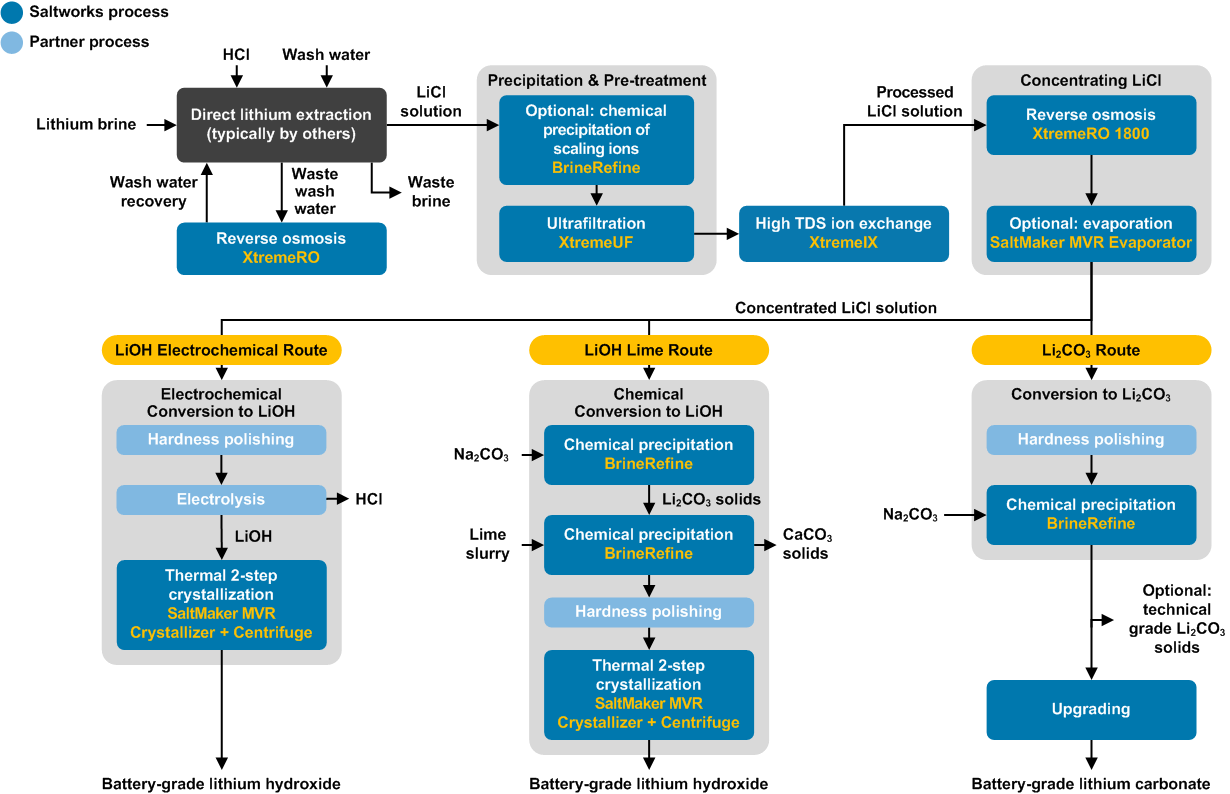
Lithium Test Center
Saltworks’ Lithium Test Center combines expertise and industry-leading technology to provide innovative solutions for processing lithium resources into battery-grade outputs. The Center is dedicated to de-risking lithium processing projects and accelerating full-scale implementation.