Cathode Active Materials (CAM) Wastewater: Recovering Valuable Minerals And Meeting Discharge Limits
A case study of treatment options and solutions for CAM wastewater
Nov 29th 2023
Article Highlights
- Lithium-ion batteries are leading the electrification of transport and rely on the cathode active materials (CAM) embedded within them.
- CAM plants produce high salinity manufacturing wastewaters that must be recycled or treated, typically containing tens of millions of dollars in value per year in lost lithium.
- Saltworks offers multiple solutions for CAM treatment to help clients meet discharge regulations and recover precious materials and minerals. Clients should consider the three key options below.
CAM Overview
Lithium-ion batteries (LiBs) are the cornerstone of global electrification. Within these batteries, lithium ions move between an anode and a lithium-based cathode, composed of cathode active materials (CAM). The main CAM in today’s commercial batteries are:
- Lithium cobalt oxide (LiCoO2)
- Lithium manganese oxide (LiMn2O4)
- Lithium iron phosphate (LiFePO4), also known as “LFP”
- Lithium nickel manganese cobalt, also known as “NMC”
LFP and NMC batteries dominate the electrification of transport. LFP batteries are known for their high cycle rates and lower cost, but they are heavier. NMC batteries are known for higher energy densities and lower weights, making them suitable for higher-range and ‘sportier’ electric vehicles—all contain cathode active materials.
Both precursor CAM (pCAM) and CAM facilities produce high-salinity blended wastewaters that are often subjected to stringent discharge regulations due to environmental and health risks, while also containing mineral value. In the context of NMC battery plants:
- pCAM plants manufacture valuable mixed metal hydroxides of Ni, Co, and Mn and produce sodium sulfate-rich wastewater.
- CAM plants combine the valuable pCAM outputs with lithium hydroxide or lithium carbonate to produce NMC battery cathode active materials and lithium-rich wastewater.
CAM and their precursor materials represent a significant proportion of a lithium battery’s value. Efficient treatment of pCAM and CAM wastewater offers the dual opportunity to meet discharge requirements while recovering valuable materials for up-processing or recycling.
Precursor CAM (pCAM) Wastewater Treatment
While the focus of this article is the treatment of CAM wastewater, pCAM wastewater deserves mention despite typically having little residual value. It predominantly consists of sodium sulfate with low levels of nickel, cobalt, and manganese. Depending on the concentration of these metal ions, metal recovery may not be economical. However, the high salinity of this wastewater prevents discharge and may cause defaulting to the need for zero liquid discharge (ZLD) treatment processes.
Unfortunately, sodium sulfate is a bulk low-cost commodity salt, and rarely worth refining for re-sale, especially at the small volumes found in pCAM wastewater.
And although sodium sulfate is an input to pulp and paper plants, waste sodium sulfate stocks in North America are set to grow alongside the battery industry and are already saturating resale opportunities. Electrochemical processes exist to convert sodium sulfate into sodium hydroxide and sulfuric acid; however, they require notable pretreatment, electrical energy, and capital investments that make their economics a challenge at the small-scale volumes being considered. It is plausible that neighboring industries could combine all sodium sulfate waste stocks at a centralized processing facility for conversion to useful chemical inputs to improve economics.
Since sodium sulfate recycling is not presently viable in most cases, waste minimization is the primary economic driver, rendering minimal liquid discharge (MLD) and ZLD the preferred processes. With that, reverse osmosis is an ideal initial MLD treatment step since high water recoveries can be achieved in RO. The RO brine must be kept warm to prevent sodium sulfate decahydrate precipitation and it can then be fed to a crystallizer.
Two Saltworks crystallizer options are available, with the first method often being the most competitive:
- SaltMaker MVR crystalizes anhydrous sodium sulfate (Na2SO4) resulting in the lowest mass of residual waste while returning distilled water (<200 mg/L TDS) and mixed salt crystals with lithium value.
- SaltMaker ChilledCrys crystalizes sodium sulfate decahydrate (Na2SO4-10H20), resulting in a high mass solid waste that can ‘melt’ at temperatures >32° C. This process can be hybridized with RO as explained here, offering an elegant ZLD method without the need to ‘boil water.’ However, the solids should be used in a subsequent or nearby process given their high weight and temperature stability issues.
Read more on the efficient treatment of sulfate wastewater now.
CAM Wastewater Background & Economics of Recovery
After pCAM metal hydroxide materials are produced they are combined with lithium hydroxide or lithium carbonate in CAM plants that serve NMC battery factories. The high salinity CAM wastewater generated carries notable dissolved lithium value. A case study is presented below for typical CAM wastewater, exploring chemistry and alternatives.
Table. 1 summarizes a typical NMC CAM wastewater. Given the salinity, lithium, and metals content, most jurisdictions will not allow sewer or environmental discharge. Treatment enables water recycling, however, the salt waste must be managed through either MLD or ZLD processes. Flow rates are often ~10-12 m3/hr, or 45-52 GPM.
Table 1: Average CAM Wastewater Chemistry (Summarized)
| Parameter | Average (mg/L) |
|---|---|
| pH | 12.75 |
| Total Dissolved Solids | 17250 |
| Lithium | 3265 |
| Iron | <75 |
| Nickel | 8.51 |
| Phosphate | 3.91 |
| Manganese | <0.75 |
| Parameter | Average (mg/L) |
|---|---|
| Calcium | <275 |
| Bicarbonate | <37.5 |
| Carbonate | 4570 |
| Hydroxide | 11850 |
| Chlorides | <150 |
| Sulfates | 4615 |
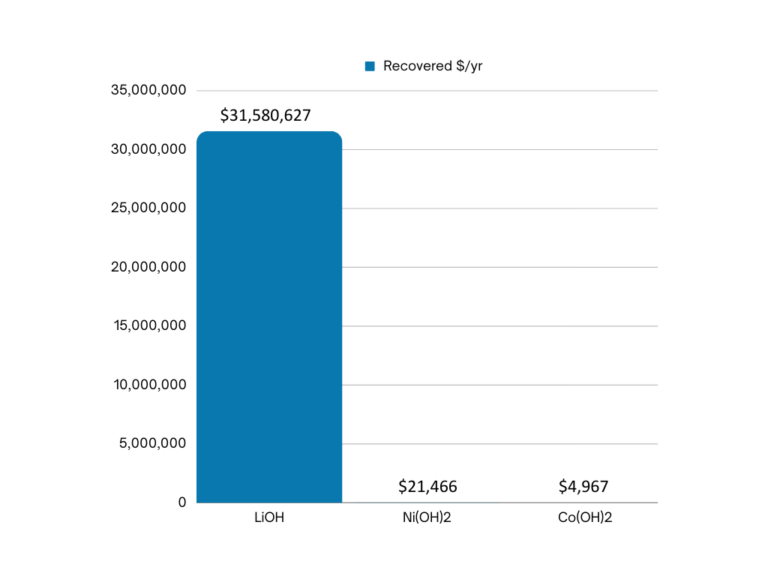
A value analysis of this wastewater, assuming 10 m3/hr. and that all metal atoms are recovered as hydroxides, reveals two important conclusions, as depicted in Figure 1 below:
- The lithium value is high and requires careful consideration.
- The metal value is low, and unlikely to pay for purification for re-use vs. safe disposal.
CAM Wastewater Treatment Options
The treatment options for CAM wastewater vary depending on the desired outcome. For simplification, this article will focus on processes that achieve the following outputs:
- ZLD and Mixed Salts Recovery: Simplest process, but lowest value outputs.
- Lithium Sulfate Recovery: Moderately complex process with high chemical inputs, but offers the most beneficial net economics.
- Low to Battery Grade Lithium Hydroxide Recovery: Most complex process, but has the highest value outputs.
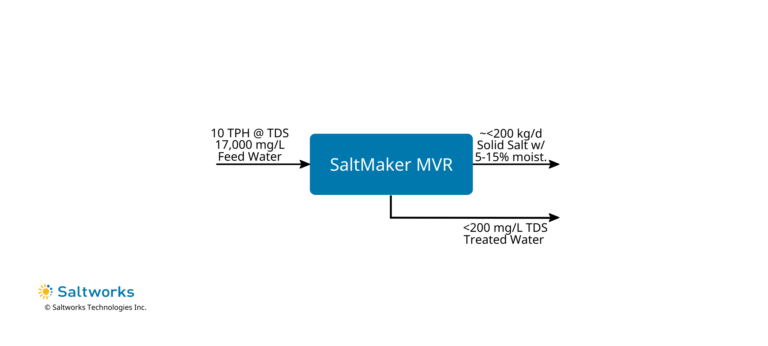
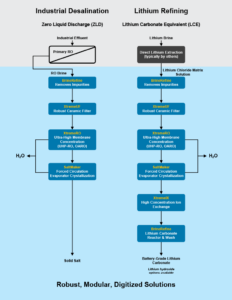
1. ZLD and Mixed Salts Recovery
- Treatment Method: ZLD evaporative crystallizer such as the SaltMaker MVR decanter to produce mixed salts.
- Treated Water Quality: <= 200 mg/L TDS. Lithium polish may be required to discharge in certain jurisdictions, however, it is recommended to consider in-plant re-use.
- Solids Disposal: Consider selling to battery recyclers or spodumene conversion plants, while understanding these solids may fetch the lowest market price owing to mixed consistency.
- Driver To Use Method: When seeking a simple plant and rapid start-up, understand that options 2 and 3 can be incorporated in the future.
- Alternative Sub-Options:
- Pretreat out metals via high pH precipitation to enhance recycle options for the mixed lithium salts.
- Practice MLD and produce a liquid brine, however significantly more mass (~4X) will need to be hauled off-site.
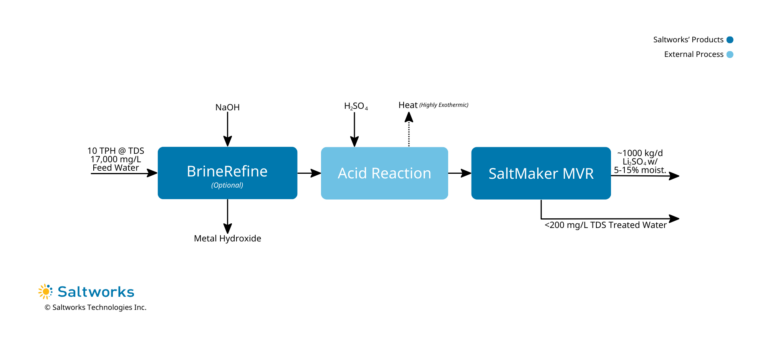
2. Lithium Sulfate Recovery
- Treatment Method: Optional precipitation of metals to improve purity through BrineRefine, while monitoring calcium sulfate precipitation, followed by acidification with sulfuric acid to convert all hydroxides to sulfates (removes the mixed carbonate-hydroxide salt issue), followed by an evaporative crystallizer, such as SaltMaker MVR, to produce lithium sulfate. An optional drier may be added.
- Treated Water Quality: <= 200 mg/L TDS. Lithium polish may be required to discharge in certain jurisdictions, however, it is recommended to consider in-plant re-use.
- Solids Disposal: Most likely sold to a spodumene conversion facility given that lithium sulfate is an intermediary chemical in their production processes and the masses involved, when blended, would have negligible impacts on production.
- Driver To Use Method: When seeking to resell the solids to a spodumene processing facility.
- Key Considerations:
- Manage the reaction time and heat generated from acidification.
- Very large volumes of sulfuric acid will be required.
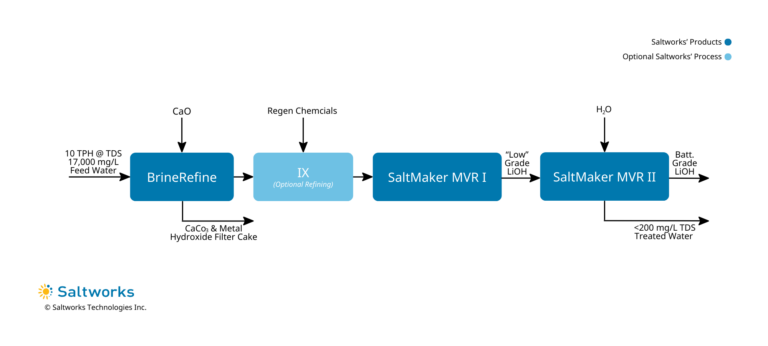
3. Lithium Hydroxide Recovery (From Low to Battery Grade)
- Treatment Method:
- ‘Liming’ of lithium salts in BrineRefine to convert all to lithium hydroxide while precipitating metals and calcium carbonate, removed from the process as a solid filter cake.
- Optional high TDS chelating ion exchange to polish out residual calcium and metals.
- Bulk crystallization of lithium hydroxide in MVR I. Unlikely to meet battery-grade specifications.
- Re-refining and re-crystallization of lithium hydroxide monohydrate in MVR II, to produce battery-grade lithium hydroxide.
- Treated Water Quality: <= 200 mg/L TDS. Lithium polish may be required to discharge in certain jurisdictions, however, it is recommended to consider in-plant re-use.
- Solids Disposal: Re-use back in the CAM plant or sell to an offsite re-refiner.
- Driver To Use Method: When seeking to recover and reuse lithium hydroxide internally in a CAM ecosystem.
- Key Considerations:
- Highest capital cost and space requirements but produces the highest value output.
- To maintain purity, lithium hydroxide monohydrate should not be exposed to the atmosphere.
Contact Our Experts
In summary, pCAM and CAM saline wastewaters need to be treated in most jurisdictions. So, when planning a pCAM or CAM facility, considering wastewater treatment ahead of time can save money compared to expensive offsite liquid disposal. Such treatment also presents an opportunity for manufacturers to offset some, if not all, of the costs of treatment by recovering lithium. In some cases, treatment can become a profit center instead of a cost.
Although each CAM plant will produce different wastewater, which will vary with time, three general treatment options exist. Saltworks’ solutions perform all three options with scalable modularity and smart digitization built into their core. All options can be staged to ‘get started’ now and ‘add value’ later, so it is important to consider and assess the options for your specific needs today and in future.
Reach out to speak with our experts and begin your CAM wastewater treatment and mineral recovery project today.
About Saltworks
Saltworks Technologies is a leader in the development and delivery of solutions for industrial wastewater treatment and lithium refining. By working with customers to understand their unique challenges and focusing on continuous innovation, Saltworks’ solutions provide best-in-class performance and reliability. From its headquarters in Richmond, BC, Canada, Saltworks’ team designs, builds, and operates full-scale plants, and offers comprehensive onsite and offsite testing services with its fleet of mobile pilots.