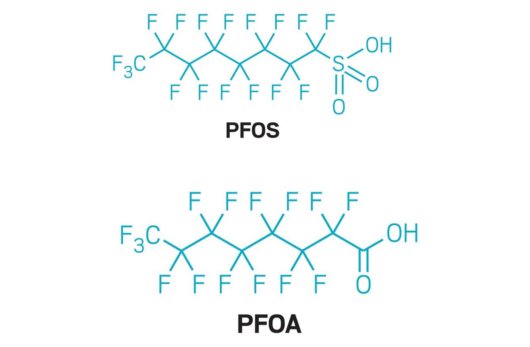
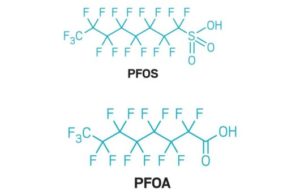
Ultra High Pressure Reverse Osmosis for Landfill Leachate
Reverse osmosis (RO) is the best available technology to treat landfill leachate for surface discharge. Possible trace volatile organic compounds (VOCs) and ammonia emerging in the RO permeate can be removed with a polishing step to meet the highest discharge standards.