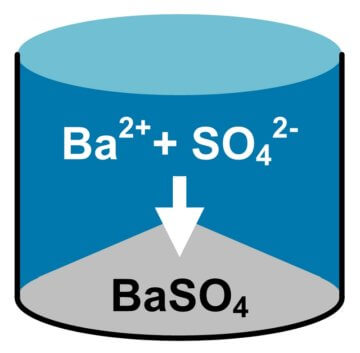
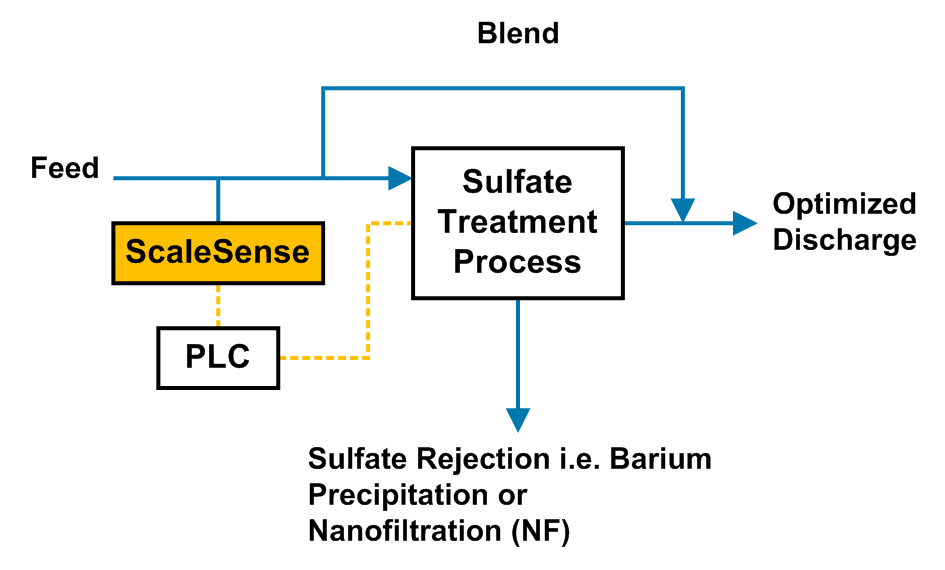
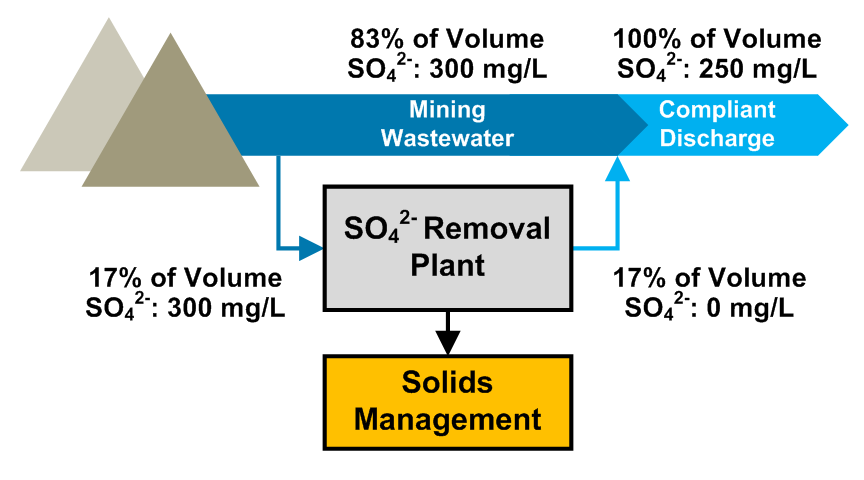
Modular & Intelligent Selective Contaminant Removal Solution Delivered
Saltworks is thrilled to help clients lower installation and operational costs and future-proof their plants. This was recently demonstrated through delivery of a modular BrineRefine and XtremeUF system that enables the targeting of ions of value or concern with substantially reduced waste.